
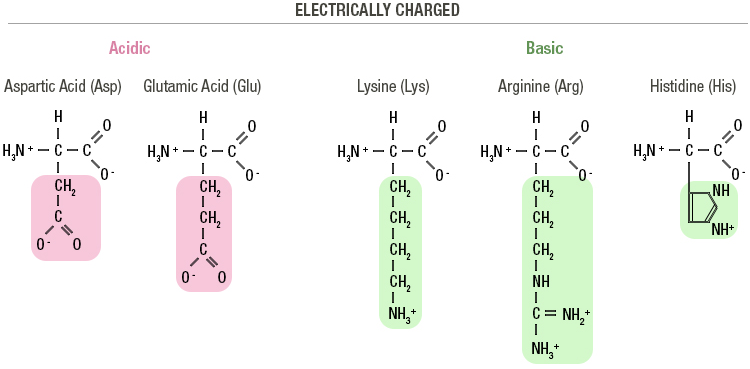
Join us on June 20th at 6pm JLM Book your place HERE Merit-based tuition scholarships (up to 100%) and living stipends available for international students accepted to M.Sc. Join our Virtual Open Day and explore the wide range of international degrees offered at our top-ranked university, known for its pioneering research, academic excellence, interdisciplinary approach & vibrant student life. The Hebrew University of Jerusalem, a global leader in multidisciplinary research, invites outstanding candidates to join our international community of world-class researchers. The Hebrew University of Jerusalem Calls all thinkers, innovators and those who dare to discover! Last call to apply for Hebrew University’s 2021-2022 graduate and post-graduate programs. While a carboxylate can act as a base, water has a pKa of 15.6, so there's no way a carboxylate will deprotonate water. If you want to deprotonate something with an x pKa value, you'll need to use a base that has a pKa which is higher than x. Your comment about the carboxylate (COO-) deprotonating water can also be addressed using pKa values. NH2 and COO-) and if you lower the pH to 3, then both the carboxylic acid and amine will be protonated (i.e. To extend this principle further, if you raise the pH to 12, both the carboxylic acid and the amine will be deprotonated (i.e. Therefore, at pH 7, amines will be protonated (NH3+) and carboxylic acids will be deprotonated (COO-). As a rule of thumb, carboxylic acids have pKa's of ~5 and amines have pKa's of ~10. Conversely, when the pH of the solution is below the pKa, the group will be in its protonated state. Very simply put, when the pH of the solution is above the pKa, the group will be in its deprotonated state. The charged state of the amine and carboxylic acid functionalities on amino acids depends on the pH of the solution, which in turn is related to the functional groups' pKa (-log Ka). In summary, RNase activity and uptake into cells are both required for E rns to act as an IFN antagonist, and the C-terminal amphipathic helix containing the GAG-binding site determines the efficiency of cell entry and its intracellular localization.Textbooks most often illustrate amino acids in their charged states at pH 7 (physiological pH).
Moreover, the C-terminal domain on its own determines intracellular targeting, as GFP fused to the C-terminal amino acids of E rns was found at the same compartments as wt E rns. Here, we show that at least three out of four positively-charged residues in the C-terminal glycosaminoglycan (GAG)-binding site of BVDV-E rns are required for efficient cell entry, and that a positively charged region more upstream is not involved in cell entry but rather in RNA-binding. The structural envelope protein E rns also exists in a soluble form and, by its endoribonuclease activity, degrades immunostimulatory RNA prior to their activation of pattern recognition receptors. E rns and N pro, both expressed uniquely by pestiviruses, counteract the host’s innate immune defense by interfering with the induction of interferon (IFN) synthesis. The genus Pestivirus, family Flaviviridae, includes four economically important viruses of livestock, i.e., bovine viral diarrhea virus-1 (BVDV-1) and -2 (BVDV-2), border disease virus (BDV) and classical swine fever virus (CSFV).


 0 kommentar(er)
0 kommentar(er)
